Advancing EV Production and Quality Control with Timegated® Raman
In the field of biotechnology, the study and utilization of extracellular vesicles (EVs) hold great promise for various applications, ranging from diagnostics to therapeutic delivery systems. EVs are nanoscale particles released by cells that contain a diverse cargo of molecules, such as proteins, RNA, nucleic acids, lipids, and metabolites. Purification and quality control of EVs are crucial for their successful implementation. Timegated® Raman spectroscopy offers many benefits in monitoring the production, quality, and diagnostic purposes of EVs.

The Finnish Red Cross Blood Service's scientists working in a laboratory. Postdoctoral researcher Heikki Saari (on the right) is participating in a project related to time-gated Raman in characterizing biological nanoparticles.
Extracellular vesicles have demonstrated their ability to play various roles in cell communication. They are involved in processes such as regulating immune responses as well as cancer metastasis. The novel mechanism of cell signaling through EVs is currently a popular topic in cell biology.
Simultaneously, there is a growing interest in utilizing EVs for diagnostics, particularly in liquid biopsies, as well as for therapeutics and drug delivery. In many diseases, the composition and quantity of EVs undergo changes, which opens up possibilities for using EVs as early diagnostic tools or prognostic indicators. Moreover, EVs can hold much of the therapeutic potential of stem cells. They could be harnessed for treating complex diseases.
- EVs come from cells, from the living organism, but they are their own nanoparticle entities, summarizes Dr. Saara Laitinen, R&D Manager of Finnish Red Cross Blood Service.

Laitinen is leading a research project EVE – Extracellular Vesicle Ecosystem for Theranostic Platforms funded by Business Finland and the 13 organizations participating in the consortium.
Overcoming Challenges in EV Purification and Yield
Challenges in exosome purification include achieving high purity and yield. Many existing, conventional methods result in the loss of some EVs during purification. Separating EVs from complex mixtures, especially blood-derived samples containing similar lipoprotein particles, poses additional challenges. Traditional monitoring methods are laborious and require multiple techniques.
- We require quite a substantial proportion, at least 10% of the manufactured sample for quality assurance and a settings analysis. If we could minimize that amount, we would end up saving time, resources, and money. All these traditional tests are quite laborious and very costly; they demand antibodies, various kinds of devices and services, and core facilities to perform them. The aim is to bypass these laborious analysis steps, Dr. Laitinen describes.
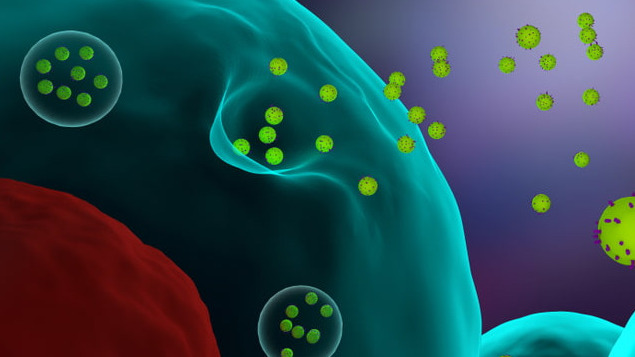 3D illustration of cells releasing exosomes. EVs consist of a variety of subtypes, including exosomes, microvesicles (MVs), ectosomes, oncosomes, and apoptotic bodies. EV is understood to be a generic term that refers to secreted vesicles.
3D illustration of cells releasing exosomes. EVs consist of a variety of subtypes, including exosomes, microvesicles (MVs), ectosomes, oncosomes, and apoptotic bodies. EV is understood to be a generic term that refers to secreted vesicles.
EV production is carried out overnight in standard conditions, but it takes almost one week to finish the analysis of the purity, yield, and other characteristic issues.
- We need to use multiple methods to assess the quality of immunoblotting to detect specific curves. We measure size distribution and nanoparticle concentration with light scattering techniques and then look at the particles’ morphology with electron microscopy. None of those methods are really that great for assessing the EV concentration accurately, she continues.
Time-gated Raman spectroscopy offers a convenient solution by providing real-time information during the separation process, thereby speeding up monitoring and reducing the need for extensive analysis.
- With time-gated Raman, you could establish how much impurity there is in relation to, for example, the lipid peak that would equate to the EV concentration. So, you can get a lot of information with one measurement, she estimates.
Monitoring Blood Bags and EV Therapeutics
The use of time-gated Raman spectroscopy in EV research and production can provide quick and non-destructive insights into molecular compositions. By examining the Raman spectra, scientists can obtain information about the composition and quality of EVs without manipulating or destroying the samples. This makes time-gated Raman spectroscopy an appealing tool for monitoring and quality control throughout the EV manufacturing process.
There are several steps which need controlling and time-gated Raman could be a solution to use for that purpose. It's quick, less sample-demanding than the conventional methods, and could be done separately from the process itself. We could see what's happening in molecular compositions without touching the blood bag, Laitinen states.
To further explore the use of Raman in extracellular vesicles characterization The Finnish Blood Service has applied for a six-month project, Novel Raman spectroscopy methods for characterizing biological nanoparticles. During the project funded by the Academy of Finland and GeneCellNano Flagship, EV measurements are carried out with the Timegated® Raman spectrometer PicoRaman M3.
- This approach involves inline monitoring of the sample flow and quality using Raman chromatography, as Laitinen describes.
The initial attempt at using a detector in line with the FPLC (Fast Protein Liquid Chromatography) system to monitor the purification process of exosome production obtained promising spectral data. The aim is to monitor the quality signals and composition of the product during storage, transportation, and other process steps without manipulating or destroying the sample.
- It seemed very easy, just tubing to link the detector to the instrument and it worked as it should. To me, it seemed like we got reasonably good spectral data even with that first attempt already. I was very pleased, explains Heikki Saari, a postdoctoral researcher in the project, and in the Finnish Red Cross Blood Service.
The research team has been investigating the application of Raman spectroscopy for monitoring blood bags and ensuring the quality control of EV therapeutics. By employing Raman measurements in a non-destructive and high-throughput manner, they seek to identify potential quality signals and specific events that may affect the composition of the products during the process steps.
Basically, something that doesn't destroy the sample, doesn't require manipulation of the sample, and can be measured through the plastic bag to get the result. And I think the first results with time-gated Raman are promising, Laitinen evaluates.
Further measurements confirmed that especially lipoproteins are visible and discovered well from the analysis with PicoRaman M3.
- Measurements were fast, and the signal-to-noise ratio was good. The results show that the spectrometer can highlight the biochemical differences in different EV samples, describes Dr. Jacopo Zini, Senior Application Specialist in Timegate Instruments.
Manufacturing EVs for Research, Therapeutic Entities, and Drug Carriers
 Digital illustration of streaming blood cells in color background.
Digital illustration of streaming blood cells in color background.
The Finnish Blood Service receives an average of 185 000 whole blood donations per year, from which blood is fractionated into different clinical-grade products such as red blood cells and platelets.
- As our mission is to create opportunities for saving lives, we pursue investigating novel therapies for life-threatening diseases, Laitinen explains.
The EV stock of the Blood Service is currently used for research purposes only, for example from diagnostics to monitoring health conditions.
- But because of our central role in Finnish health care, we are also investigating the possibility to develop a platform for using EVs as therapeutic entities and drug carriers in future applications, she describes.
Particularly, they are developing a platform for red blood cell-based EVs, where pure red blood cells are isolated and transformed into EV-like structures called nanoerythrosomes. These structures can be loaded with various components, such as small molecules, enzymes, and RNA. By modifying the targeting moieties on the EV surface, they aim to enhance the therapeutic potential of EVs.
- EVs are like stories with different compositions that can tell us about certain things. We already have different cell types used for clinical purposes, and EVs are expected to be the next big thing in therapeutic applications. However, there are also certain issues with quality assurance and control when it comes to producing these cells, Laitinen expects.
Different types of cells such as red blood cells, platelets, nucleated cells, and immune cells are used in various applications. For some new therapies, it's crucial to determine which cells are suitable for patients. Immune cells, for example, can't simply be separated from blood and given to all patients due to the careful tissue typing needed. A monitoring solution may be necessary for also these diverse cell products.
- If we could use the Raman signal to correlate certain signals with specific parameters from other analyses, that would be the jackpot. This would eliminate the need for manual sample collection while still obtaining the required information, she continues.
In the first stage, the group is planning to manufacture EVs from red blood cells and platelets on a larger scale globally.
- Possibly in the future EVs could be produced from all different cell types in the blood, including immune cells. Maybe someday, instead of the entire cell, we could treat patients with EVs from the cells that are produced from cell factories, carrying specifically needed target moieties. Hopefully, and maybe already in the near future, we could be producing differently labeled EVs required for nanoscale devices as reference material for clinical flow cytometry or other quality control analysis.
The EV Market Expansion
 3D rendering of coronavirus RNA strand.
3D rendering of coronavirus RNA strand.
Due to the pandemic and the RNA vaccines, the legislators were forced to re-evaluate the rate of new medicine process progression.
- Developing new medicines was taking far too long and especially in this living cell area. Companies won´t survive for such a long period of time. This is an evolving area, and many different aspects influence market development, Laitinen evaluates.
Another important issue from the global blood services perspective is monitoring for potential new blood transmittable viruses or microbes spreading.
- Currently, we are taking certain routine tests on every donated blood case, and we are following potential threats very carefully. Novel and unknown viruses form an area where sensitive enough monitoring tool would be highly appreciated to detect already in the very early phase whether there's a yet unknown virus in the bloodstream, Laitinen figures.
A few years ago, discussions around EVs were questioning the characterization of these particles, as they are not considered traditional medicine or pharmaceutical compounds with their origin lying in living cells.
- They don't have a nucleus, so they could be considered much safer than the entire cell of an Advanced Therapeutic Medicinal Product, but it is not a straightforward topic. First in line is safety, then comes efficacy. Components whose origin comes from living cells’ compatibility with tissues, blood, and even cross-species need to be the first to consider. It is understandable that regulation and legislation are progressing a couple of steps behind, and this field moves forward at different rates on different continents, she continues.
The global exosomes market size was valued at 112.25 million USD in 2022. It is anticipated to expand at a compound annual growth rate (CAGR) of 32.75% reaching 1.03 billion USD by 2030, according to the Grand View Research.
- The estimates about the size of the EV market have been a bit overheated during the last ten years, and still, it´s very risky to predict this area and the timeline of the expected results especially in clinical use. But if we look at patenting and publications, the amount has increased exponentially during the last five years.
The challenges in predicting the progress of the EV market derive from the heterogeneity of EVs and the purity to produce them from different origins.
- How well can we manufacture these in a pharmacologically safe way, is the big question. The focus is on pharmacological and composition quality, and that´s the reason manufacturing purification enrichment issues like yield and purity are crucial. We are still in an early phase in the scientific understanding of what EVs are.
Solving Global Issues with Therapeutics
 Extracellular vesicles are nanoscale particles released by cells that contain a diverse cargo of molecules.
Extracellular vesicles are nanoscale particles released by cells that contain a diverse cargo of molecules.
Recently discovered, a new important factor to be analyzed from EVs is functionality.
- When developing therapies, we should study not only the characteristics but the functionality of the sample as well. You might have all the pieces there and you can see them in a vitro analysis, but the compositions might not be in a functional format. That is again in relation to how the EVs are manufactured and purified, stored, transported, and recovered for further use, and do they still have their original functionality, Laitinen explains.
Large-quantity manufacturing of EVs for therapeutic use requires a quality control system that doesn't waste too much of the samples.
In that sense, all the other analyses should be carried out in a less sample-consuming way. Time-gated Raman might be useful in blood panel studies for characterizing lipid profiling diagnostics, for example, reducing the number of samples that you must analyze further.
The EVs are also challenging to study because of their multidimensional composition.
- I see the EV area with even more potential than we originally saw gene therapy. The challenge and the opportunity are two sides of the same coin. There are multiple different EVs even in one pool, all these different sources of EVs in different cell types. EVs can be used as such or enhanced, loaded, or modified versions. We can actually learn from EVs.
Currently, the Finnish Blood Service has estimated to be able to produce a maximum amount of four platelet products per week directed to EV production. Billions of EVs are available from one concentrate.
- One concentrate ends up in multiple products, and all those products in this R&D purpose can help scientists. We aim to keep the price as low as possible to ensure accessibility also for researchers. Our goal is to make high-quality products with reasonable prices to accelerate the field. It's a scientific advancement, so in the future, we could be in a position where we also have a therapeutic device that saves lives.
Read a blog that discusses EVs composition and classification, biological function and medical interest, and the use of time-gated Raman technology in EV analysis.
Sources used in the article:
Interview with Saara Laitinen and Heikki Saari
The University of Helsinki, Extracellular vesicles description (https://www.helsinki.fi/en/researchgroups/extracellular-vesicles).
Grand View Research: Exosomes Market Size, Share & Growth Analysis Report, 2030 (https://www.grandviewresearch.com/industry-analysis/exosomes-market).
Author
 The writer is Timegate Instruments' Marketing Director Elina Aronen-Raappana. Elina has M.A. in Science Communications and she is an Executive MBA Candidate. Read more about her and the whole Timegate team.
The writer is Timegate Instruments' Marketing Director Elina Aronen-Raappana. Elina has M.A. in Science Communications and she is an Executive MBA Candidate. Read more about her and the whole Timegate team.