Spiking: A Tool for Faster Model Calibration in Biopharmaceuticals
The benefits of Raman spectroscopy as a process analytical technology (PAT) tool in the field of biopharmaceuticals are well known: it’s fast, non-destructive, and label-free. Indeed, Raman spectroscopy has become a well-established tool for quality control in upstream biopharmaceutical processes, where soft chemometric modeling is used to interpret spectral measurements in real-time and produce predictions on a variety of variables, most commonly concentrations of key analytes like glucose or lactate. This information can then be used to optimize other bioreactor parameters like the perfusion rate or feed schedule, ultimately leading to better and more stable yields.
The Foundation is in the Quality of the Training Data
The foundation of any chemometric model is the quality of its training data. Ideally, for a soft chemometric model, the training set should consist of a large number of spectra and their corresponding reference concentrations, where the concentration varies over a range representative of that expected in the target application. Additionally, for a robust model, the training set should encompass likely sources of variation in the process. This could mean changes in the bioreactor materials, environmental factors like temperature or humidity, or even personnel changes.
The Challenges of Soft Chemometric Models in Bioprocessing
Often a training set for a soft chemometric model will be compiled from process data resulting from several bioreactor runs representative of the target application of the model. In theory, this should ensure that the training data resembles that to which the model will be applied, thereby improving model accuracy. This also has the benefit of ensuring samples at relevant concentrations are included in the training set. However, this method is also time-consuming, expensive, and laborious on account of the duration of the bioreactor runs and the amount of reference analysis needed. Furthermore, in a bioreactor variation in one analyte’s concentration will often correlate with changes in another component’s, creating a risk that the given analyte’s effect on the spectra is not isolated by the model.
Bioreactor Sample Spiking as an Alternative Strategy
One alternative strategy that addresses these concerns is to utilize sample spiking. Spiking refers to the practice of taking an existing sample and adding to it a known quantity of a given analyte. This practice can be utilized in various ways to expedite the creation of a training set. For example, the samples taken from a bioreactor run can be spiked with varying and known amounts of some analyte and then measured again offline using a Raman spectrometer. This allows control over the variation in the analyte in isolation from other changes and increases the number of spectra in the training set without additional reference analysis.
Leveraging Controlled Mixtures for Acquiring Training Data
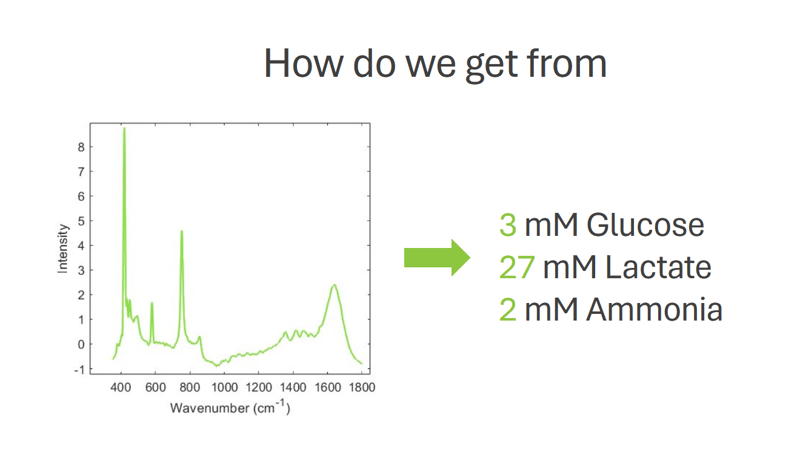
In some cases, it’s possible to forgo process samples completely when acquiring training data. Instead, mixtures are created with known and varying proportions of relevant bioreactor components and then measured using the Raman spectrometer to complete the training set. These mixtures would include, for example, glucose, lactate, ammonia, viable cells, and feed agent. Of course, process data will always be needed to validate the model once built, but this approach provides complete control over the variation in concentration on the training set and reduces the demand for reference analysis, thus reducing the time and investment needed to acquire sufficient training data.
Towards Efficient Biopharmaceutical Research
When effective, less resource-heavy strategies for chemometric modeling like these have the potential to bring flexibility and speed to biopharmaceutical research via more efficient process analytics. Progress in this area could eventually lead to the increased accessibility and affordability of biopharmaceutical products. Timegated® Raman tools show potential in enhancing chemometric modeling in biopharmaceutical manufacturing. Discover more about Timegated® Technology and how it can transform your biopharmaceutical processes!
Read our previous blog post on soft and hard chemometric modeling in biopharmaceutical manufacturing for more insights.